Most acquired, partial auricular defects have a good surgical solution. The more superior on the ear the defect is located, the more choices there are for reconstruction. Reconstruction of the lobule is the most difficult and is aesthetically the most important.
Although some defects can be closed by soft tissue alone, cartilage is usually required for support. For smaller defects, a conchal cartilage graft may suffice. However, for larger defects the rules of firmin (Firmin, Personal communication, 2013) are extremely helpful: defects that consist of 25% or more of the helical rim orinvolve more than two planes (i.e., involve antihelix as well as helix and scapha) will require rib cartilage for support. Conchal cartilage will not provide sufficient support in these cases.
External Auditory Canal. Stenosis is best treated by a full-thickness graft applied over an acrylic mold, provided a reasonable recipient vascular bed can be prepared. Occasionally, multiple Z-plasties are used to relieve webbing of the orifice, or a local flap is employed to line the canal and break up the contracture. an acrylic stent is recommended for several months to counteract the inexorable tendency toward contracture.
Helical Rim Acquired losses of the helical rim vary from small defects to major portions of the helix. The former defects, which usually result from tumor excisions or minor traumatic injuries, are best closed by advancing the helix in both directions, as described by Antia and Buch 1 (Figure 27.1). The success of this excellent technique depends first on freeing the helix from the scapha via an incision in the helical sulcus that extends through the cartilage but not through the posterior skin. The posterior auricular skin is undermined, until the entire helix
is hanging as a chondrocutaneous flap on the posterior skin. Extra length can be gained by a V-Y advancement of the helical crus, as described in the correction of the constricted ear. defects up to 1.5 cm can be closed without tension. Defects larger than 2 cm are too large for this technique. In order to facilitate closure, it is necessary to “cheat” by removing some of the scaphal cartilage, taking tension off the reapproximated helical rim. Reducing the scapha reduces the size of the ear and the patient should be alerted to this fact in advance. Although originally described for upper-third auricular defects, this technique is also effective for middle-third defects, as well as for defects at the junction of the middle and lower thirds.
If the helical rim alone is missing, as may occur in burn injuries, a thin tube of retroauricular skin can be applied to the residual scapha with acceptable results (Figure 27.2). This is one example where cartilage may not be necessary. The disadvantage of this technique is that it requires three stages to “waltz” the tube into place: (a) formation of the tube in the sulcus, (b) transfer and insetting of one end of the tube, and (c) transfer and insetting of the other end of the tube.
Upper-Third Defects. Techniques available for upper-third defects in increasing order of size and complexity are as follows (Figure 27.3):
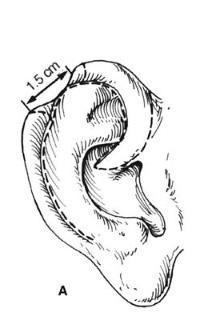

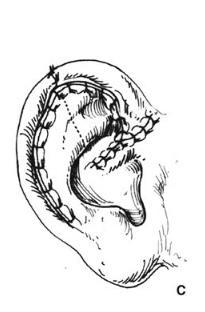
Figure 27.1. Antia-Buch helical advancement. a. An incision is designed inside the helical rim and around the crus of the helix. B. The incision is made through the skin and the cartilage, but not through the posterior skin. The helical rim is advanced to allow closure and a dog-ear of skin (dotted line) is removed on the back of the ear. c. Closure showing the crus of the helix advanced into the helical rim. (Copyright Charles H. Thorne, MD.)
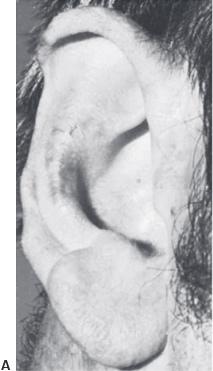


Figure 27.2. Helical reconstruction with a thin caliber tube flap. a. Burn deformity of the helix. B. Construction of the tube flap in the retroauricular sulcus. c. Transfer of one end of the tube. d. Final result. (Courtesy of Burt Brent, MD.)
Cartilage grafts can be inserted via the Converse tunnel procedure in which the skin is not detached at the junction of the residual ear and the retroauricular skin. The problem is that precise placement of the graft with exact coaptation

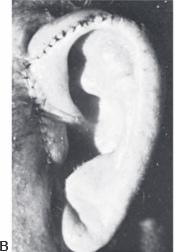



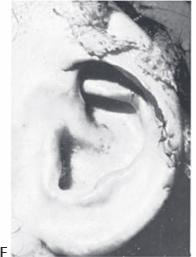


Figure 27.3. Four techniques for repairing upper-third auricular defects. a and B. Preauricular flap. The flap is transposed to repair a minor rim defect. c and d. Antia-Buch helical advancement. e and f. The combination of a retroauricular flap and conchal cartilage graft. G and H. Chondrocutaneous conchal flap to reconstruct the helical rim. Of the upper-third techniques, the only one not shown is a rib cartilage graft, which is shown in Figure 30.11. (Courtesy of Burt Brent, MD.)
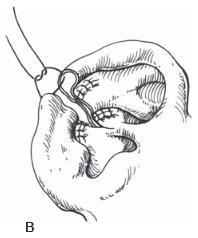
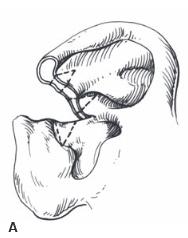
Figure 27.4. Wedge resection and primary closure with excision of accessory triangles. A Wedge excision performed and accessory triangles designed. B. Closure of the defect. The accessory triangles help prevent the auricle from cupping forward. (Copyright Charles H. Thorne, MD.)
to remaining cartilage is difficult using this approach, and a detached retroauricular flap (Figure 27.5) is preferable. Middle -third auricular tumors are excised and closed by either a wedge resection with accessory triangles (Figure 27.4) or a helical advancement, as previously described.
lower-third auricular defects. SVarious techniques have been described to reconstruct earlobe defects using soft-tissue flaps. These techniques are not as effective as those that employ cartilaginous support. Like the alar rim, the normal earlobe does not contain cartilage. A reconstructed earlobe, however, will only maintain its contour if cartilage is included,analogous to nonanatomic alar rim grafts. The author prefers to use thin, flat cartilage obtained from the nasal septum.2 The cartilage is placed beneath the cheek/retroauricular skin in the first stage. A hole can be made in the cartilage at this initial stage for later ear piercing. In the second stage, an incision is made around the cartilage graft and the flap is advanced beneath the earlobe as in a facelift (Figure 27.6).
Microtia literally means small ear. The simplicity of the term belies the vast complexity of this entity, in terms of both the variable clinical presentation and the difficulty of surgical reconstruction.
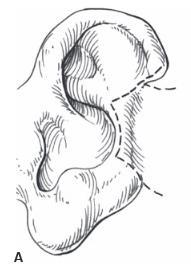

Figure 27.5. Wedge resection and primary closure with excision of accessory triangles. A Wedge excision performed and accessory triangles designed. B. Closure of the defect. The accessory triangles help prevent the auricle from cupping forward. (Copyright Charles H. Thorne, MD.)
Gillies is credited with the first use of rib cartilage for con-struction of an auricular framework in 1920. The importance of his contribution was temporarily obfuscated by several reports using allogeneic cartilage. The allogeneic cartilage, whether from a living donor such as the patient’s parent or preserved cadaver cartilage, always underwent gradual resorption.
The modern era of auricular reconstruction began with Tanzer 3 who reintroduced the technique of autogenous costal cartilage grafts as a method of auricular reconstruction. Tanzer’s results inspired Brent who modified, improved, and standardized a four-stage technique of auricular reconstruction. 4 Nagata developed a more complex technique that condensed microtia repair into two stages.5 The Nagata technique requires more cartilage and the construction of a higher profile, more detailed framework than the Brent technique. Firmin analyzed those characteristics of a “Brent ear” that fall short of a normal ear and reported a large series using her modification of the Nagata technique.
While the technique of autogenous auricular reconstruction was evolving, silastic was also used, instead of rib cartilage, as the auricular framework. This material, as well as other artificial materials, led to a high incidence of extrusion. More recently, the use of porous polyethylene frame-works has been explored and has become the standard treatment offered by some surgeons. The largest series was reported by Reinisch.7 Early attempts were associated with a 42% incidence of framework extrusion leading to modifications of the original technique and coverage of the framework using a temporoparietal fascial flap. This drastically reduced the complication rate and is the technique of choice in his opinion.
Finally, an auricular prosthesis is another option. The introduction of titanium osseointegrated fixtures by Branemark has made prosthetic reconstruction of the auricle a more stable and user-friendly alternative. 8 The role of prosthetic reconstruction in microtia will also be discussed later.
The ear is composed of a delicate and complex-shaped cartilage framework covered on its visible surface with thin, tightly adherent, hairless skin. A reconstructed auricular framework must be more rigid than the cartilage framework of a normal ear. When the auricular framework is placed beneath the skin in the temporal region, a combination of the tight skin envelope and the progressive scar contracture will gradually obliterate the fine details if the framework is built to mimic the delicate framework of the normal ear. as such, any reconstructed ear that maintains its projection and definition in the long term will be more bulky and will lack the flexibility of the normal ear.
Consequently, even the best result using current techniques for auricular reconstruction is imperfect. The deficiencies of current techniques make it even more important that the reconstructed auricle be the correct size, be located in the proper position such that one earlobe is not higher than the other, and be properly angulated relative to the other facial structures.
The middle and external ears are derived from the first (mandibular) and second (hyoid) branchial arches. Most patients with microtia have atresia (absence) of the external auditory canal and tympanic membrane with variable deformities of the middle ear ossicles. Rarely, a patient will present with microtia and a patent, stenotic canal. Least common but most difficult to repair are patients with an auricular vestige and canal that are markedly abnormal in position. Because
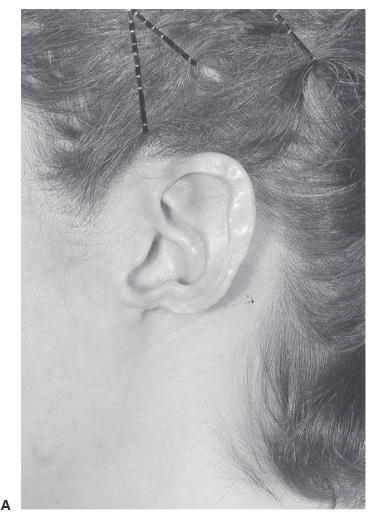

Figure 27.6. Earlobe reconstruction using nasal septal cartilage. a. Original defect secondary to discoid lupus erythematosus. B. Final result after two-stage reconstruction using thin cartilage from the nasal septum. (Copyright of Charles H. Thorne, MD).
the meatus can only be moved a limited distance, the surgeon must consider complete excision of the canal. The inner ear is derived from totally separate embryologic tissues from the middle/external ear and is, therefore, almost always normal in patients with microtia. In other words, the hearing loss in microtia/atresia patients is conductive in nature
The incidence of microtia varies widely among ethnic groups. Textbooks cling to the figure of 1 in every 6,000 births. The incidence is higher in patients of Asian ethnicity. In addition, microtia is almost twice as common among males as females and almost twice as common on the right side compared with the left. Bilateral microtia occurs somewhere between 10% and 20% of patients with microtia.
Most cases of microtia occur in an isolated fashion. Only rarely does microtia appear to run in families. One exception is Treacher Collins syndrome, which frequently presents with bilateral microtia and is inherited in an autosomal dominant fashion.
Older publications suggest that isolated microtia and hemifacial microsomia are distinct entities. In fact, microtia is part of the spectrum of hemifacial microsomia deformities, all of which owe their origin to maldevelopment in the first and second branchial arches. At one end of the spectrum is the patient with microtia who appears to have an otherwise symmetrical face. At the other end of the spectrum is a patient who manifests underdevelopment of all tissues on one side of the face, including microtia, aural atresia, underdevelopment of the mandible, underdevelopment of the soft tissues of the cheek, and underdevelopment of the facial nerve. Microtia and hemi - facial microsomia should not be considered as separate enti - ties (Chapter 24).
Patients with unilateral microtia/atresia usually have normal hearing in the contralateral ear. This should be verified by an otologist as early as possible after birth. The main goal then becomes protection of the better hearing ear throughout development. It is important that otitis media in the ear with normal hearing be treated completely and that a hearing test be repeated after completion of treatment. Residual middle ear fluid in the normal ear may result in hearing impairment and consequently interference with speech development.
Patients with unilateral microtia reasonably do well from a hearing/speech point of view. They have difficulty localizing sounds and discriminating sounds in noisy environments. Patients with unilateral atresia can frequently function in a classroom and traditionally have survived without amplification. School performance, however, is improved if the child/ teacher employ an FM unit or, even better, if the child has a bone-conduction or bone-anchored hearing aid to provide binaural hearing.
Patients with bilateral microtia/atresia are in an entirely different situation. These patients are functionally deaf with complete conductive hearing loss bilaterally. These patients are fitted with a bone-conduction hearing aid as early as pos - sible in life and benefit from a bone-anchored hearing aid retained with a titanium abutment when they get older.
Approximately one-half of the patients with microtia/aural atresia have middle ear anatomy that can be reconstructed surgically. In bilateral cases, this is extremely important and may eliminate the need for a hearing aid or at least decrease total dependence on such a device.
The issue in the unilateral case is not as clear because, as stated above, these patients function reasonably well. Most otologists around the world do not recommend canaloplasty
in patients with unilateral microtia . The surgical results are prone to stenosis of the external auditory canal meatus as well as scar contracture of the reconstructed tympanic membrane. While the immediate postoperative audiograms show excellent results, the hearing in the reconstructed ear tends to deteriorate with time. There are otologists, however, who believe that the results of canaloplasty are more than sufficient to warrant the procedure in unilateral cases. The timing of the auricular reconstruction relative to the canaloplasty is important. the auricular reconstruction is best performed before the canaloplasty . Auricular reconstruction is possible after canal surgery but the result is compromised by the scarring in the region.
The microtia deformity itself is enormously variable. At one end of the spectrum is an auricle that is slightly small but otherwise normal in appearance. At the other end of the spectrum is the patient with complete anotia. Various classifications have been proposed to deal with the vast variability in clinical presentation. The Nagata classification is useful because it correlates with the surgical approach
The following are the three options for reconstruction of microtia:
Autogenous Reconstruction. The two main techniques described for autogenous reconstruction of the auricle using a rib cartilage framework are the Brent technique and the Nagata technique.
The Brent technique involves four stages:
The Nagata technique is performed in two stages:

Figure 27.7. Fabrication of ear framework from rib cartilage. Brent technique, stage 1. a. The base block is obtained from the synchondrosis of two rib cartilages. The helical rim is obtained from a “floating” rib cartilage. B. Carving the details into the base using a gouge. c. Thinning of the rib cartilage to produce the helical rim. d. Attaching the rim to the base block using nylon sutures. e. Completed framework

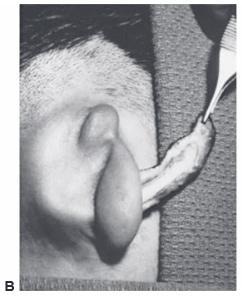

Figure 27.8. Insertion of the ear framework. Brent technique, stage 1. a. Preoperative markings indicating the desired location of the framework (solid line) and the extent of the dissection necessary (dotted line). B. Insertion of cartilage framework. c. Appearance after the first stage. A suction catheter is used to suck the skin into the interstices of the framework. (Courtesy of Burt Brent, MD.)
Technical Details of the Two Techniques. SThe patient is examined standing and the location of the earlobe on the normal side is transferred to the affected side. This is the single most important marking because symmetrical earlobes is one of the primary goals of the procedure. If the reconstructed ear is too low, it will not be aesthetically pleasing, no matter how beautiful it is in isolation. The normal ear is traced on clear x-ray film and sterilized. Using this tracing, additional templates are made. A template of the desired framework is made, approximately 3 to 4 mm shorter and narrower than the eventual ear. If the Nagata technique is performed, additional templates are constructed of the antihelix/triangular fossa piece and the tragus/antitragus piece.
The exact location and orientation of the desired auricle are drawn on the patient. Decisions are made about the location of the incisions. In the Brent technique, an incision is designed that can be used again at the time of lobule rotation and at the time of tragus construction. If the Nagata technique is used, the incision is designed as shown in Figure 27.13, to allow rotation of the lobule. The incision is made and the cartilage remnant is removed, carefully preserving the skin and avoiding buttonholes if possible. The pocket is dissected
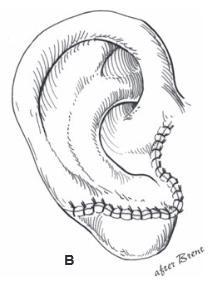

Figure 27.9. Rotation of lobule. Brent technique, stage 2. The ear-lobe is rotated from its vertical malposition into the correct position at the caudal aspect of the framework. a. Design of lobe rotation is made such that the same incision can be used in stage 4, tragus construction. B. After rotation of the lobule. (Copyright Charles H. Thorne, MD.)
beyond the outline of the eventual auricle. In the Nagata technique, a pedicle is maintained to the dissected flap to improve blood supply.
Attention is turned to the chest. Although a transverse incision will heal more favorably than an oblique incision, the latter provides better exposure. The rectus abdominis muscle is divided. In the Brent technique, two pieces of cartilages are harvested. In the Nagata technique, five pieces are required. In addition to the synchondrosis of two cartilages and a free rib for the helical rim, the Nagata technique requires removal of a piece for the antihelix/triangular fossa, a piece for the tragus/ antitragus, and a piece to be banked in the chest for the second stage. This piece is wedged into the sulcus at the second stage to provide projection of the auricle. Nagata harvests the cartilages in a subperichondrial plane, leaving the perichondrium in the chest when the cartilages are removed. The author tends to take the cartilages with the perichondrium and has not noticed a significant difference in the chest wall deformity. If a pneumothorax is created, a catheter is placed into the pleural cavity. After the incision is closed the catheter is withdrawn while the anesthesiologist applies positive pressure ventilation. An additional catheter is left in the wound for the administration of Marcaine postoperatively.
Details are applied to the base using gouges. In the Nagata technique, the antihelix/triangular fossa piece is attached. The helical rim is attached in a similar fashion in both techniques. The difference is that Nagata recommends waiting until the child is 10 years old, which yields cartilages that are long enough to reconstruct the crus of the helix. Finally, the tragus/ antitragus piece is attached in the Nagata technique. Nagata uses wire sutures. The author has used nylon sutures, rather than wire, for both the Brent and Nagata techniques, with adequate fixation and a low incidence of suture extrusion.
The framework is inserted into the pocket along with two suction drains. Once the closure has been accomplished and the dressing has been applied, the drains are attached to Vacutainer tubes. The tubes are changed every half hour for 2 hours, then every hour for 2 hours, and then every 4 hours overnight. The dressing is removed on the second postoperative day and the patient is discharged.
Firmin has made significant modifications in the Nagata technique and has now accumulated the largest experience with experience with ear reconstruction in the worldover 3,500 cases. The modifications will not be discussed in detail because she has not yet published them but they must be recognized as her contributions.9 In most cases, she employs a simpler incision than Nagata that preserves


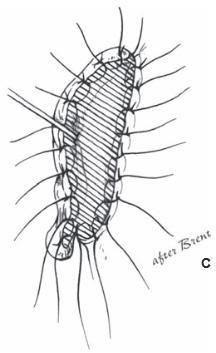
Figure 27.10. Elevation of framework and skin graft to sulcus. Brent technique, stage 3. a. Incision is designed behind the ear. B. The retroauricular scalp is advanced into the sulcus so that the eventual graft will not be visible. c. Full-thickness graft to the exposed medial surface of the auricle. (Copyright Charles H. Thorne, MD.)
the retrolobular skin and increases the likelihood that the patient can have the ear pierced in the future. In addition, she has added additional pieces of cartilage on the deep surface of the framework to increase the projection of and stabilize the tragus and to increase the height of the posterior conchal wall. She has also developed classifications and algorithms for the management of the skin, the type of framework necessary, and the technique used for the elevation at the second stage.
An example of framework construction is shown in Figure 27.15. The appearance after insertion in the skin pocket is shown in Figure 27.16. An example of a postoperative result is shown in Figure 27.17.
complications. Complications of the Brent technique are rare in experienced hands. Complications of the Nagata technique, at least in the author’s hands, are relatively common. The most common complication is exposure of the cartilage framework. Management requires experience, but these wounds may heal by secondary intention if they are less than 1cm in maximum dimension and not over a prominent part
of the framework. Exposed areas of more than 1 cm in greatest dimension require urgent coverage, usually with a temporoparietal flap and skin graft. In some cases, a flap of skin from the retroauricular region may be used to cover a small area of exposed helix. For areas of exposure over the antihelix, a flap of conchal skin can be rotated, leaving the concha to be skin grafted. In fact, if there is the slightest question about whether an exposed area will heal, then flap coverage is indicated. one never regrets performing flap coverage of an exposed area of cartilage framework, but one may certainly regretnot performing such a procedure.
elevation of framework. In the third stage of the Brent technique and the second stage of the Nagata technique, the previously placed framework is elevated and the retroauricular sulcus is resurfaced. Nagata adds a piece of rib cartilage covered with a temporoparietal flap. The cartilage is banked under the skin at the time of the first stage and is wedged into the sulcus to provide projection to the reconstructed auricle in the second stage. The fascial flap covers the graft and provides a bed for skin grafting (Figure 27.14). In both the techniques

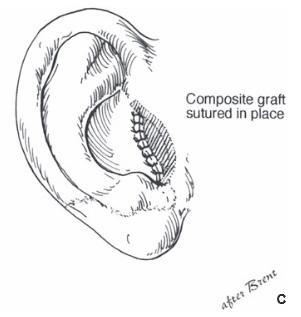
Figure 27.11. Construction of tragus. Brent technique, stage 4. a. The conchal graft is taken from the posterior conchal wall of the contralateral ear. B. An L-shaped incision is made and the graft is inserted with the skin surface down. c. The graft healed nicely. (Copyright Charles H. Thorne, MD.)

Figure 27.12. Fabrication of ear framework from rib cartilage. Nagata technique, stage 1. a. In a manner similar to Brent, the base and its details are carved from the synchondrosis of two adjacent ribs. B. The four pieces of cartilage that make up the cartilage framework are seen and numbered. The base and helical rim are present as they are for the Brent technique. There is an additional antihelix-triangular fossa piece and an additional tragus-antitragus piece that are unique to the Nagata procedure. (Copyright Charles H. Thorne, MD.)
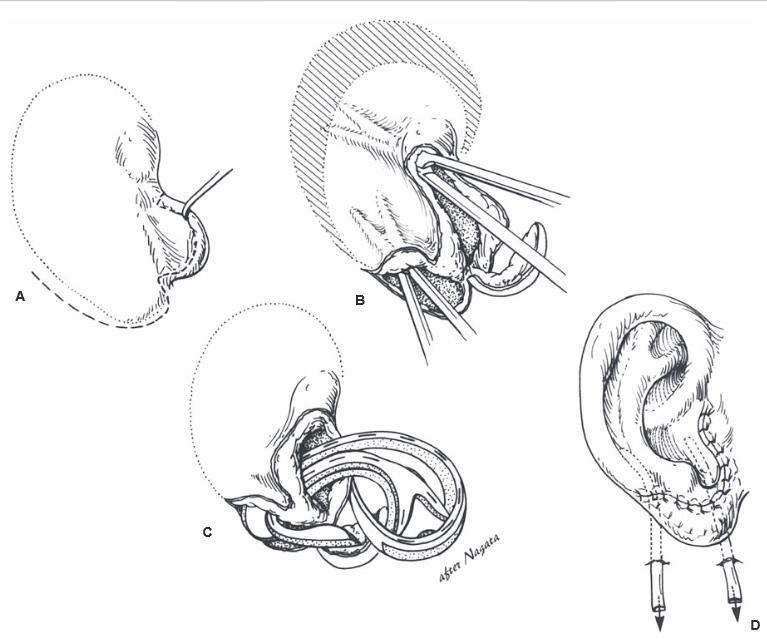
Figure 27.13. Insertion of the cartilage framework. Nagata technique, stage 1. a. The incision is designed, robbing most of the skin on the medial surface of the lobule that will be necessary to line the concha. B. The pocket is dissected,leaving an intact “pedicle” at the caudal end of the flap. c.The framework is inserted. d. Appearance of the framework after stage 1. Suction drains are in place to coapt the skin to the underlying cartilage. (Copyright Charles H. Thorne, MD.)


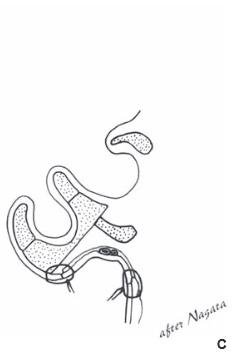
Figure 27.14.Elevation of framework. Nagata technique, stage 2. a. The auricle is elevated, the scalp is advanced into the sulcus (arrows), the cartilage graft is wedged into the sulcus, and the graft is covered with a temporoparietal flap and skin graft. B. The skin graft is in place. Nagata described the use of split-thickness skin but this author has noted tremendous shrinkage of the thin grafts and recommends full-thickness graft. c.Cross section showing the cartilage graft in place providing projection as well as the temporoparietal flap covering the cartilage graft. (Copyright Charles H. Thorne, MD.)
the scalp is advanced into the depth of the sulcus, and the medial surface of the elevated framework is resurfaced with a skin graft
Both Nagata and Brent recommend a split-thickness graft for this stage. The grafts contract significantly, however, in
some cases obliterating the reconstructed sulcus. For this reason, the author prefers a full-thickness graft from the groin. The disadvantage is a visible scar but the full-thickness graft resists contracture and is more likely to result in maintenance of the reconstructed sulcus.

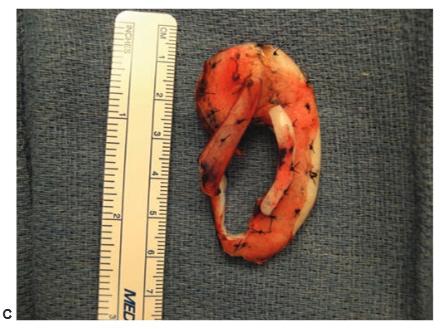
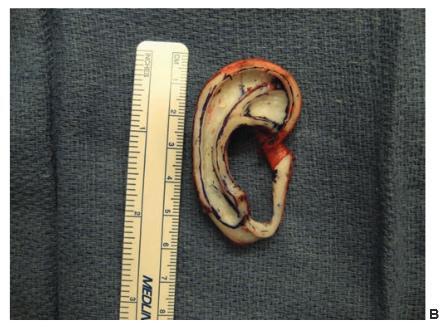
Figure 27.15.Construction of the cartilage framework. a. This author uses Nylon sutures to attach the cartilage pieces to construct the framework. Firmin and Nagata employ stainless steel wire. B. The completed framework. c. The deep surface of the completed framework showing the extra pieces described by Firmin to stabilize the tragus and to increase the projection of the posterior wall of the concha. (Copyright Charles H. Thorne, MD).


Figure 27.16.The framework immediately after insertion. a.Appearance of the framework after insertion and application of suction to the drains. B. Close-up view. The abnormal contour near the lobule is one of the drains. (Copyright Charles H. Thorne, MD.).
composite Autogenous/Alloplastic Reconstruction.In these patients, an auricular framework composed of porous polyethylene (Medpor) is used instead of costal cartilage. Reinisch originally reported a 42% incidence of implant exposure. He modified the technique, adding temporoparietal flap coverage of the framework, and reported a vastly decreased complication rate.
Prosthetic Reconstruction.Prior to the introduction of implant retention of prostheses, prosthetic reconstruction depended on adhesive retention and was impractical. Branemark osseointegrated titanium implants have made prosthetic reconstruction somewhat more practical but this technique remains, in the author’s opinion, a second choice to autogenous reconstruction.
children are poor candidates for prostheses, often refusing to wear them regardless of the retention mechanism. Children
also tire of the maintenance required of the abutments and the surrounding soft tissue. If adequate hygiene is not maintained, the skin/abutment interface becomes inflamed and use of the prosthesis must be discontinued awaiting resolution of the inflammation. Additionally, the daily removal and replacement of the prosthesis serves as a constant reminder of the deformity. In contrast, children with an autogenous reconstruction incorporate the new ear into their sense of self. Finally, prostheses lack the warmth and texture of autogenous reconstructions and, despite the superior details, are not more “lifelike.”
It is important to note that prostheses require replacement every five years for the life of the patient and, therefore, prosthetic reconstruction is more expensive in the long term than autogenous reconstruction.
To this author’s thinking, the only absolute indication for prosthetic reconstruction in a child with microtia is failed


Figure 27.17.Example of a patient with microtia and the postoperative result. (Copyright Charles H. Thorne, MD.).
autogenous reconstruction with inadequate soft tissue for either a second autogenous reconstruction or a Medpor reconstruction. In such a patient, a prosthesis may represent the only salvage procedure available.
Relative indications for the use of prosthetic reconstruction include a very low hairline where a temporoparietal flap would be required to allow autogenous reconstruction or extreme hypoplasia of the tissues with a concavity where the auricle will eventually be located.
Personal Thought on Surgical Reconstruction.The author has extensive experience with both the Brent and the Nagata techniques of auricular reconstruction and it is on the basis of that experience that the following comparative statements are made.
The Nagata procedure was designed to address the perceived weaknesses of the Tanzer/Brent technique, particularly the region of the concha, crus of the helix, tragus, and incisura intertragica. As such, the best possible Nagata-type result may have superior details to the best possible Brent-type result. The problem is that the “best possible results” do not occur most of the time.
The Nagata procedure, at least in the hands of this author, is definitely associated with a higher complication rate. The framework is of much higher profile, is much more complex in its details, and contains many more sutures. As such, the chance of cutaneous necrosis with framework exposure is significantly greater using the Nagata technique. On the other hand, these areas of exposure are generally small and heal without further surgical intervention and do not necessarily compromise the result.
The individual surgeon must decide, factoring in his/her experience, whether the possibility of a superior result is worth the increased risk of the Nagata procedure. In his own practice, this author currently uses the Nagata/Firmin technique in most patients. In patients with extremely tight skin, or the presence of other scars, the Brent technique is used because of its safety and reliability.
The other issue involves the chest donor site. The Nagata technique requires harvesting twice as much cartilage as the Brent technique. While Nagata harvests all cartilage subperichondrially, no detailed study has been performed comparing the chest wall deformity created by the Nagata technique at age 10 years with the deformity created by the Brent technique at age 6 years. Although the donor site is an issue not to be ignored, it tends not to be an issue regardless of which technique is used. Patients simply do not complain about the chest unless they are extremely thin.
Proponents of the composite alloplastic/autogenous reconstruction using Medpor cite the lack of chest donor-site scars/ deformity as an advantage. Although that is true, these same reports fail to mention the scars/deformity that replaces the chest deformity. For example, the composite technique robs the contralateral normal ear of all the skin behind it, resulting in obliteration of the sulcus or a skin graft donor-site scar if the retroauricular defect is replaced with partial-thickness skin. Additionally, this technique requires a scalp scar to harvest the temporoparietal flap. These scars are frequently hypertrophic and/or associated with thin strips of alopecia, which may be more troublesome to the patient than a chest wall scar.
Placing the reconstructed ear in the best location is straight forward if the face is symmetrical or near symmetrical. In cases of significant asymmetry, however, compromises must be made. The surgeon cannot rely on measurements from landmarks such as the lateral canthus and oral commissure, because the entire side of the face is so much smaller than the other side. If such measurements were
used, the ear would be placed far too posteriorly and would appear strikingly abnormal. Of equal importance, however, the ear must not be placed too low or too anterior. The author attempts to place the ear in the correct craniocaudal position so that the earlobes are at the same level and then determines the anteroposterior positioning based on the relationship to the sideburn. No ear will look normal unless there is a sideburn in front of it.
Total auricular reconstruction of the acquired deformity differs from congenital microtia. There is always less skin available. In microtia, removal of the cartilaginous remnant provides some supple, unscarred skin to supplement the retroauricular skin. In the acquired situation, there may be no residual ear skin, and the presence of scarring from the traumatic or surgical removal of the ear restricts the skin pocket. In many cases, a temporoparietal flap with skin graft is required in addition to the native skin. The flap provides an unlimited amount of vascularized tissue, but the combination of the flap and the skin graft never has the definition or color match of the native skin. In addition, the presence of an external auditory meatus limits the access incisions, the extent of the skin pocket, and the risk of infection. The canal is colonized with bacteria, frequently Pseudomonasspecies, which adds additional problems not encountered in microtia cases.
A hematoma may result from trauma and frequently occurs in wrestlers. Unless evacuated, the blood tends to become cartilaginous, resulting in the so-called cauliflower ear. Once fully developed, the cauliflower ear is extremely difficult to correct. Hematomas may require repeated aspirations or an incision to fully evacuate. Suturing gauze bolsters to the auricle to compress the skin against the cartilage usually prevents reoccurrence (Figure 27.18).
Most attempts to replace an amputated ear will fail, resulting in additional incisions/scars and “burning bridges” that may be useful for secondary reconstruction. The patient, however, will not easily accept the decision to discard the amputated part without an attempt at replacement. There is no easy answer.
Replantation of amputated ears has been reported and some excellent results have been obtained. The vessels are small, however, and failure is common. Any attempt at replantation must consider that success is unlikely and may result in scars that limit later reconstructive attempts. Incisions for the exposure of recipient vessels are kept to a minimum.
Reattaching large pieces of auricular tissue as composite grafts is doomed to failure. The good news is that such an attempt does not disrupt the surrounding tissues, does no harm, and makes the patient feel that “something” is being done.
Removing the skin from the cartilage and burying it beneath the retroauricular skin is a poor choice. The thin, delicate cartilage will not maintain its shape sufficiently against the forces of scar contracture. An alternative is to cover the de-skinned cartilage with a temporoparietal flap. The esthetic result will be poor for the reasons mentioned above and this useful tissue will not be available for secondary reconstruction.
Several successful cases have been reported in which the posteromedial skin was removed from the amputated part, the cartilage was “fenestrated,” retroauricular skin was excised, and the part was placed on the healthy bed. The anterolateral auricular skin is vascularized through the

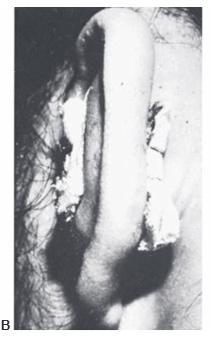
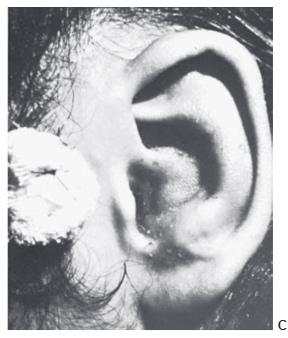
Figure 27.18. Management of an acute othematoma. A. Recurrent conchal hematoma. B. Through-and-through bolster sutures, after evacuation of the hematoma. C. Appearance of ear after the compression dressing has been removed at 10 days. (Courtesy of Burt Brent, MD.)
cartilage fenestrations by direct contact with this healthy, vascularized bed.
In the opinion of the author, the ideal scenario for an amputated ear is an attempt at microvascular replantation through the available wound, without additional incisions. If unsuccessful, secondary reconstruction with rib cartilage grafts is performed, with or without a temporoparietal flap. If replantation is not an available option, the part should be replaced as a composite graft (knowing it will fail), or the part should be discarded.
Acute burns may result in chondritis. Characterized by tenderness, erythema, warmth, and induration, chondritis usually occurs several weeks after the initial injury. Once chondritis is diagnosed, aggressive steps are taken to eradicate the infection and prevent subsequent deformity. Drainage and placement of an irrigation system is an appropriate first step. If this therapy fails, the involved cartilage must be debrided. When the latter becomes necessary, incisions are planned judiciously to minimize the effect on secondary reconstruction.
Cutaneous malignancies of the helical rim can be excised and closed with helical advancement as described above. Lesions in the concha or over the antihelix can usually be excised and skin grafted. If the cartilage is involved, it can be excised and the graft placed directly on the posterior skin. Malignant melanomas should be excised with the same margins as melanomas of the equivalent depth in other parts of the body. Melanoma in situ does not require a full-thickness excision. These lesions are excised with a 5-mm margin, preserving the perichondrium, and the skin grafted. Invasive melanomas of the helical rim require wedge resection to achieve adequate margins, eliminating helical advancement as an alternative for closure. These defects may be large and require secondary reconstruction.
While ingenious techniques have been described to reconstruct traumatic clefts in the lobe caused by earrings, the most reliable method is to excise and close the defect in one stage and re-pierce the ears 6 weeks later, or whenever the induration subsides.
Another complication of earrings is keloid formation. Small keloids can be excised and closed primarily and may not recur. If the patient is truly prone to keloids, then excision, triamcinolone injection, and pressure earrings are warranted. If the keloid recurs, excision with immediate irradiation offers the best chance of avoiding recurrence.
Finally, piercing through the cartilage in the upper portion of the ear can result in severe infections. While not common, chondritis can lead to severe, permanent disfigurement of the auricle. Infections, therefore, are treated aggressively. If cartilage requires debridement, it is performed early to limit the deformity and incisions are planned carefully to minimize these deformities.

Dr. Thorne is the Editor-in-Chief and the author of several chapters in Grabb and Smith's PLASTIC SURGERY, 7th Edition.
Ear Construction Chapter in PDF